Bio-Fire Isn't As Tough As You Think
Taylor
0
83
2023.11.30 22:32
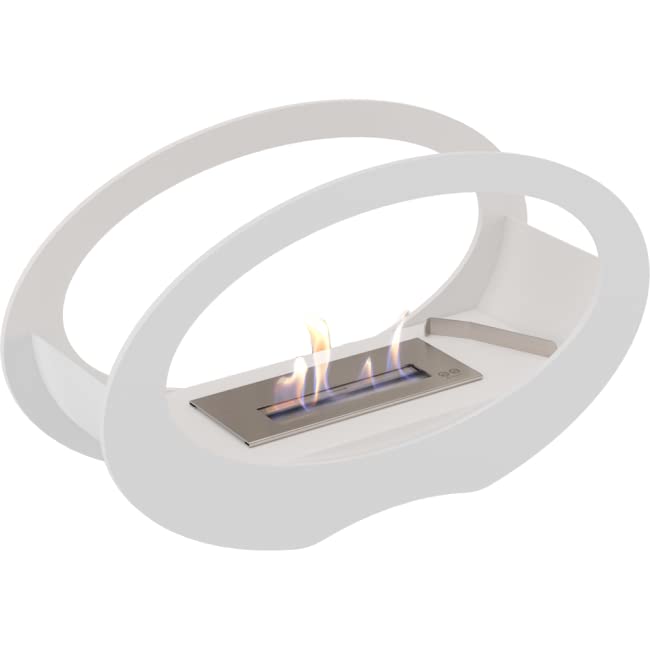 Bio-Fires - An Eco-Friendly Alternative to Real Fires
Bio-Fires - An Eco-Friendly Alternative to Real FiresBiofires are a warm and cosy way to heat your home without releasing harmful gases or soot. They are easy to install and operate - just like an electric fire.
The BARDA-funded contract enables the development of the BioFire Emerging CoV Panel, which is a nucleic acid Amplification test to be used with BioFire FilmArray Systems to detect human coronaviruses, both emerging and known, in nasopharyngeal and oropharyngeal sw and saliva specimens.
Eco-friendly
A bioethanol fire can be an excellent alternative to wood or gas fires. The fuel is made from plant by-products and uses an organic process to convert sugars into alcohol. It is a better choice over other fireplaces as it does not emit harmful emissions and has less carbon footprint. Bioethanol is a safer alternative to other types of fireplaces because it doesn't produce smoke or soot. It also does not require a flue, which means it can be used anywhere in your home or garden.
A multi-drug resistant (MDR) bacteria is among the most serious health threats of our time. New testing methods can detect MDR bacteria early and help prevent any further complications. BioFire FilmArray is one such test. This test uses a symptom-driven approach to detect common pathogens that cause sepsis. The results are available in an hour so that providers can start treatment immediately.
The process of Molecular PCR amplifies DNA and RNA molecules to millions or billions of copies. This allows for the analysis of small samples of body fluids or tissues to determine if specific genetic sequences are present. It is a useful instrument for lab professionals and can assist them to identify serious illnesses in a cost-effective and efficient way. The BioFire film array PCR system is a revolutionary instrument for diagnosing infectious diseases. It can be utilized in many locations, including hospitals and laboratories.
In the case for tests of your respiratory system, you could apply a swab of alcohol to your nose prior to sending it to the lab. BioFire’s system allows clinicians to react quickly and with a short turnaround time. This means that there is less waiting for results and better patient outcomes.
The Chanouf Farm-Biofire company developed an Agroforestry waste recycling system, that makes use of olive and pear tree waste to produce organic charcoal and biomass energy. The products are offered to the public to replace traditional wood charcoal and coal briquettes with eco-friendly alternatives. These bio-waste products are also suitable to be burned in a Kachelofen fireplace that produces radiant heat and is a more sustainable alternative to electric or gas fires.
Clean burning
When you utilize a bio fire you aren't just reducing energy consumption, you're also helping the environment by using a sustainable fuel source. This is because the combustion of hanging bioethanol fireplace does not produce harmful gases such as carbon dioxide. It only releases heat and water vapour. This makes it a safer and cleaner alternative for indoor use.
Ethanol is produced by fermenting sugars in plants. It is a renewable source of fuel. This means it can be made quickly and won't run out like fossil fuels like oil and coal. The use of bioethanol can also help reduce the dependency on imports from foreign countries and reduces the risks of supply shortages.
The bio-fires can be installed easily within existing fireplace openings or as a standalone unit. This makes them a convenient alternative to wood and gas fires. They are an excellent choice for homes and Bio Fuel Fires offices that do not have a chimney.
When a bio-fire is ignited, the burner is fitted with a special media that absorbs the liquid ethanol fuel and makes it accessible to be burned. The ethanol fuel is burned in the flames, and the smoke are released into the air through the vent. This makes the flames appear more natural and creates an amazing beauty that cannot be replicated with wood or gas fires.
bio ethanol table top fire-fires benefit from not requiring vents or flue to replenish oxygen used during combustion. They also don't produce carbon monoxide which must be vented into the room in gas fires with open fronts.
The BioFire Respiratory Panel utilizes multiplex technology that simultaneously tests for 19 respiratory pathogens in about an hour. This lets doctors identify and rule out respiratory illness more quickly than conventional tests that require visits to the patient. Both patients and the health system benefit from this.
Safe indoors
Bio-fires are safer to use indoors than real fires, since they produce no smoke or carcinogenic fumes. They also don't emit carbon monoxide, which can cause death if breathed in. The bio fuel fires (visit the site)-ethanol fuel used in them is flammable however you must take great care when handling the burner and refilling your fire. The fuel should only be added after the burner has been switched off and the fire has cooled. Any spills need to be cleaned immediately.
The company's diagnostic FilmArray system--BioFire Torch and BioFire 2.0--are user-friendly and able to run eight different diagnostic panels that have been cleared by the US Food and Drug Administration and three additional panels that are approved for emergency use and six environmental test panels. These tests include a respiratory panel that was developed to quickly recognize and distinguish respiratory illnesses such as COVID-19. emerging coronaviruses and COVID-19.
The Biomedical Advance Research and Development Initiative, which is part of the US Department of Defense Strategic Access Program has granted the COVID-19 panel a grant. The COVID-19 test is an antigen-based system, allowing clinicians to distinguish between symptoms caused by COVID-19 and other respiratory illnesses with a similar degree of accuracy as the current rapid antigen tests being used.
The company is also developing an SARS-CoV-2 test to be used in its Respiratory Panel 2.1. The multiplexed nucleic acid amplification test will be available on the BioFire filmArray 2.0 and Torch Systems and will detect SARS CoV-2 and 21 respiratory pathogens using saliva and nasopharyngeal samples within less than an hour. The test will be developed within the shortest timeframe, and FDA approval for EUA and the 510(k) is expected to be granted in Q3 2020.
bio ethanol fireplace insert-ethanol flame effect fires are safe to use, but should be kept clear of combustible items such as pillows, curtains and blankets. You should never place them close to anything that could catch biofuel fire. Keep flammable materials away from the flame-effect fires by at least 10 feet. The bioethanol fuel must be stored in a dry, secure space that is not easily accessed by children or pets.
Simple to use
The bioethanol fireplace does not have a chimney to be concerned about. You will require a space to store it as well as fuel. Once you've filled it and lit it, ignite it. This will create a flame that looks just like an actual fire. Once the flame is lit, it will warm the room without much smoke. It also produces heat similar to the wood-burning fireplace. Bio fires are easy to maintain and don't need any servicing, just the odd clean-up.
built in bioethanol fireplace contrast to other tests that are quick that are available, the BioFire RP2.1-EZ test uses an syndromic approach to detect common symptoms of infectious diseases and returns results in about 45 minutes. The test is for patients with upper-respiratory symptoms and has a specificity rate of 98%. The test can identify up to 19 viruses and three bacteria in a single sample which makes it a good choice for patient management.
The BioFire system integrates multiplex PCR testing, sample preparation, and reporting of results into one integrated platform. This helps laboratories to streamline their workflow and eliminates the requirement for a sequential process that involves individual and send-out test. This could lead to improved patient outcomes by enabling the correct test to be administered first time, and by eliminating the need for costly tests based on culture. The BioFire system can identify pathogens at a individual level and also their antimicrobial resistance gene. This information is more reliable than conventional methods.
Molecular diagnostics is becoming increasingly utilized to improve patient outcomes and treatment. The BioFire panel RP2.1+ employs a syndromic method to simultaneously target 23 pathogens that are frequently implicated in respiratory infections. The test is available on the BioFire film array 2.0 and Torch systems and provides results in 45 minutes.
In the US the US, this test is currently available under FDA Emergency Use Authorization (EUA), and will be commercially available after the initial DoD demand is met. Additionally the BioFire Defense Team has developed a fully-automated, sample-to-result assay to quickly identify of COVID-19. This assay is in development and will be available once the initial DoD demands are fulfilled.
